Electron Shells and Bonding
The electrons in an atom are arranged
in Shells around the nucleus. These
shells are also known as electron
Orbital or Energy
Levels. The Lowest energy level is always
filled first - the most inner shell. Each shell can hold only a limited
number of electrons The first shell can only hold
2 electron - lowest energy level. The Second shell up to 8 electrons. The Third can also hold up to
8 electrons etc. Atoms with their outer shell full
of electrons, do not react with anything. They are very stable. Most atoms do not have their outer
shell full of electron and this is what makes them want to react Other
then the NOBLE
GASES, the atoms of all other
elements have incomplete outer shells. That is why they react. For any atom to
become stable it must
have its outer shell full of electrons 8 of the with the exception of Hydrogen
and Helium, which only require 2 electrons. They can do this in two different ways: By losing or gaining electrons or
- ionic bonding By sharing electrons - covalent bonding Ionic Bonding - it is all about atoms losing or gaining electrons to become stable,
i.e. (have their
outer shell full of electrons). Remember ! A
full outer shell makes an atom stable. During ionic bonding,
atoms gain or lose electron(s) to form stable negatively (-Ve) or positively (+Ve) charged ions which are then strongly
attracted to one another to
form an IONIC
BOND. Ionic
bonds form between metals and non-metals. Most metallic elements lose electrons to form a POSITIVE
ION. Most non-metallic elements gain electrons to form a NEGATIVE
ION. An ION is a charged particle (not an atom any more). It is charged because it contains an unequal number of protons and electrons. Let's look at sodium chloride as an example:
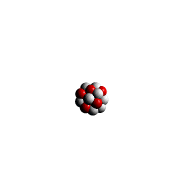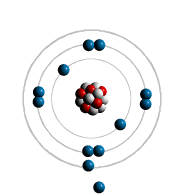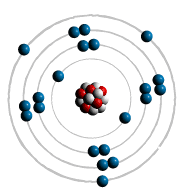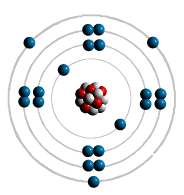
Atomic Bonding:
Some facts:
Water Bond:
Tags:Water bond, Ionic bond, Atomic bonding, Electron Shells, Bonding, Atomic bonding, how do atoms bond, ionic bonds form between molecules that have, chemical bonds ionic bonds, how are ionic bonds form, electron bond, ionic bond, covalent bond